In South Africa, producers and public are faced with poor water quality in most areas. This is brought about by degrading filtration systems, over populated areas adjacent to productive farmland, to name but two factors.
Irrigation water quality is a critical aspect of greenhouse crop production. There are many factors which determine water quality. Among the most important are alkalinity, pH and soluble salts. But there are several other factors to consider, such as whether hard water salts such as calcium and magnesium or heavy metals that can clog irrigation systems or individual toxic ions are present. In order to determine this, water must be tested at a laboratory that is equipped to test water for agricultural irrigation purposes.

Poor quality water can be responsible for slow growth, poor aesthetic quality of the crop and, in some cases, can result in the gradual death of the plants. High soluble salts can directly injure roots, interfering with water and nutrient uptake. Salts can accumulate in plant leaf margins, causing burning of the edges. Water with high alkalinity can adversely affect the pH of the growing medium, interfering with nutrient uptake and causing nutrient deficiencies which compromise plant health.
Reclaimed water, runoff water, or recycled water may require reconditioning before use for irrigation since disease organisms; soluble salts and traces of organic chemicals may be present.
Water quality should be tested to ensure it is acceptable for plant growth and to minimize the risk of discharging pollutants to surface or ground water.
Quality Filters
Suspended solids need to be removed from water to prevent clogging of piping, valves, nozzles and emitters in an irrigation system. Suspended solids include sand, soil, leaves, organic matter, algae and weeds. Ground water, although usually clean, may contain fine particles of sand or other particulates. All of these can be removed through filtration. Before selecting a filter, a water analysis should be done.
pH and Alkalinity
Alkalinity and pH are two important factors in determining the suitability of water for irrigating plants. pH is a measure of the concentration of hydrogen ions (H+) in water or other liquids. In general, water for irrigation should have a pH between 5.0 and 7.0. Water with pH below 7.0 is termed “acidic” and water with pH above 7.0 is termed “basic”; pH 7.0 is “neutral”. Sometimes the term “alkaline” is used instead of “basic” and often “alkaline” is confused with “alkalinity”.
Alkalinity is a measure of the water’s ability to neutralize acidity. An alkalinity test measures the level of bicarbonates, carbonates, and hydroxides in water. These compounds get into the water from the geologic materials of the aquifer from which the water is drawn, such as limestone and dolomite
Adverse effects on nutrition
In most cases irrigating with water having a “high pH” causes no problems as long as the alkalinity is low. High pH water has little effect on growing medium pH because it has little ability to neutralize acidity.
Of greater concern is the case where water having both high pH and high alkalinity is used for irrigation. The problem is most serious when plants are grown in small containers because small volumes of soil are poorly buffered to pH change. Therefore, the combination of high pH and high alkalinity is of particular concern in plug and seedling trays.
Trace element deficiencies such as of iron and manganese and imbalances of calcium (Ca) and magnesium (Mg) can also result from irrigating with high alkalinity water. To determine if a pesticide is affected by high pH or high alkalinity, carefully review the product’s label. A call to the manufacturer may be needed to find the information for some chemicals.
Adjusting Alkalinity with Acids
Acidification of water having high pH but low alkalinity is rarely necessary, but many greenhouse operators inject acid (e.g., phosphoric, nitric, or sulfuric acid) into water with problematic high levels of alkalinity. The use of acid injection should be considered very carefully for several reasons. It is an extra step in production which will require additional materials and equipment and are dangerous to handle and may damage some injectors and piping systems.
Calculating the Amount of Acid to Use
It is suggested to use enough acid to reduce water alkalinity to within a target range. You can have a lab test your alkalinity or you can use a kit to measure it yourself (alkalinity test kits can be purchased through greenhouse or scientific supply distributors). Then, calculate the amount of acid needed to get the water into your target alkalinity range. (Current alkalinity – desired alkalinity = alkalinity to be neutralized).
Fertilizer Compatibility
Many growers want to use one injector and mix acid with fertilizers. The use of sulfuric, nitric and citric acid is compatible with most water-soluble fertilizers. Phosphoric acid is not compatible with calcium-containing fertilizers like calcium nitrate or formulations like 15-0-15 and 17-0-17 in concentrated form.
Remember, you only put in some of the acid to carry out the calibration run (half volume of stock solution). Add the remainder of the acid for the total amount of acidified water you wish to make. You may add more water, allowing “room” for fertilizer addition. Add the fertilizer carefully to avoid splashing, and add enough water to attain your final volume – mix thoroughly.
Before investing in any treatment system, however, it may be advisable to investigate the possibility of switching to an alternate water source, or mixing water sources, if it is an economical alternative for solving a water quality problem.
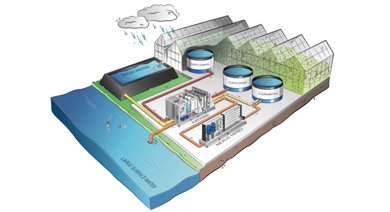
Blending with Rainwater and Other Non-Problem Water
Rainwater can be collected from roof runoff structures, such as greenhouses, where it is then stored in a cistern to be used as irrigation water. Collected rainwater could also be blended with problem waters such as those with high alkalinity, high EC, or excess Na and Cl or to improve the quality of recycled tail water and industrial wastewaters with high nutrient content used for irrigation. Other non-problem sources of water could also be used for blending.
Rainwater should be collected from clean, well-maintained structures free from mineral contaminants such as zinc and other metals. Water should be tested for pH and minerals at least twice a year.
By: Bartok, J.W. et al, UMass.


One Response