The term ‘feeding water’ is used to describe an untreated water source that is available to prepare nutrient solutions for soil-less crop production. Different factors can be used to define feeding water quality, but the chemical composition and the presence of potentially dangerous micro-organisms need special attention.
Looking at the chemical composition, firstly the concentration of ions, measured as EC, can be used as indication of the potential quality of feeding water. Water with a low EC can be used to grow any crop. High EC feeding water, usually high in sodium (Na+), magnesium
(Mg2+), sulphate (SO42-) and chloride (Cl-) can only be used to grow saline-tolerant crops.
These include amaranths, Swiss chard, melon and cherry tomatoes. Examples of crops that are extremely sensitive to saline conditions are blueberries, disas, anthurium, cymbidium and roses. Most of the remaining greenhouse crops vary between moderately sensitive to moderately tolerant, as can be deducted from the EC levels, associated with the nutrient solutions as recommended for different crops.
It should be kept in mind that the absorption of water is restricted at increased root zone EC levels. The water in the lake of Galilee is widely used in Israel, even though it has an EC of ±1.0 mS cm-1. The EC of water in the Vaal Dam varies between 0.3 and 0.9
mS cm-1 but the EC in the lower Vaal River may be higher in relatively dry seasons.
Compared to this, the quality of Stellenbosch’s water is excellent, with an EC of about
0.1 mS cm-1, as is also found in other water streams from unpolluted, high rainfall mountain areas.
However, there is no guarantee that water with a low EC can safely be used for soil-less crop production, since micronutrients may be present at phytotoxic levels without affecting the EC. Two examples are high zinc (Zn) levels in rainwater gathered from galvanised roof surfaces and high copper (Cu) levels where copper water pipes are used.

Macronutrients in feeding water
Water should be chemically analysed in order to determine the levels of the different nutrients in solution. In low rainfall areas, high levels of salts are usually present. Apart from Na+ and Cl-, high levels of essential nutrients such as calcium (Ca2+) magnesium (Mg2+) and sulphate (SO42-) may also be present in high-EC water. The other essential ions are usually found at lower concentrations, depending on the area and the water source. The higher the ratio of useful ions compared to Na+ and Cl-, the better the potential of the water.
These essential nutrients should be topped up to optimum levels. Since Mg2+ may be present at high concentrations in high-EC water, it may reach toxic levels when Mg fertilizers are simply added to the water at normal recipe levels. A high Mg2+ concentration in a nutrient solution may restrict the uptake of Ca2+ and K+.
Micronutrients in feeding water
Apart from high sodium, chloride or macronutrient levels in high-EC water, it may also contain high or toxic micronutrient levels. Micronutrients are usually present at such low concentrations that even at relatively high micronutrient concentrations they do not affect EC readings. Should feeding water contain micronutrients at high levels exceeding the concentrations prescribed for different crops, the water should be avoided or handled with care. As with macronutrients, the micronutrient levels in feeding water should be considered when planning nutrient solutions and should be topped up to optimum levels.
Micronutrient phyto-toxicity
Tomatoes can tolerate B at levels of up to 1.1 mg L-1, almost four times higher than the recommended level. It is recommend that Zn be used at 0.33 mg L-1 for substrate-grown tomatoes, but toxicity can be expected at only twice this concentration.
High Zn-levels are usually found in water gathered from galvanised roof surfaces.
Copper-sulphate is a well-known chemical, used to kill algae in swimming pools. Thus, the potential phytotoxic effect of high Cu-levels is well-known. Most crops need Cu at 0.05 mg L-1. According to Steiner (1984), Cu may be phytotoxic when its concentration
is doubled to 0.1 mg L-1. Copper pipes should thus be avoided in hydroponic units.
Manganese toxicity problems may develop on lettuce (open or loose tulip shaped heads) where seedlings are grown on sphagnum peat, due to high levels of Mn in this European substrate. High iron (Fe) and manganese (Mn) levels in some feeding water sources may block irrigation drippers. These water sources should be treated to lower Fe and Mn levels.
Total alkalinity (pH)
The pH of water and nutrient solutions can be manipulated by adjusting the total alkalinity. Total alkalinity is the aggregate concentration of carbonate, bicarbonate and hydroxide. The total of these ions (CO32- & HCO3- & OH-) in water is determined with a titration, by adding acid to the sample until the pH reaches a level of 4.5. Some water-soluble fertilizers may be slightly acidic.
Thus, the total alkalinity of the water should not be too small to prevent the pH from dropping when these fertilizers are dissolved. In mountainous, high rainfall areas, low-EC water may be acidic with a total alkalinity of zero. In order to remove the corrosiveness of such a feeding water, a huge reservoir can be filled with limestone pebbles (Aquastab) to let the water pass though it (Figure 1).
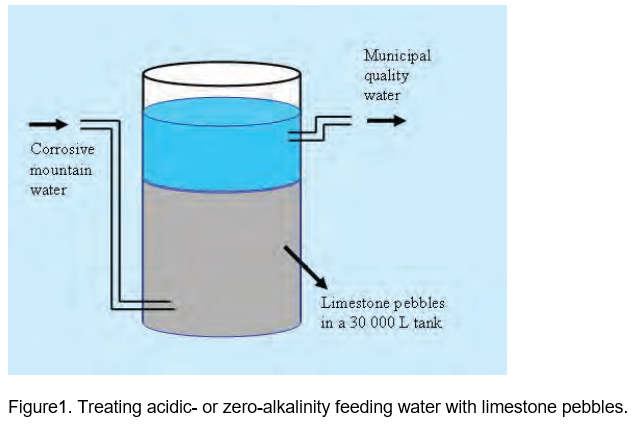
Municipalities use this technique to treat corrosive water in order to protect their water pipes. Since the solubility of limestone is very low, almost insoluble, it only adds a very small alkalinity to the water while it will also neutralize acids that may be present in the water. The water coming from a lime-pebble tank will be suitable to be used for normal greenhouse crops. Should a corrosive, low-EC water be treated with limestone pebbles, it is possible that its pH may rise to a level higher than 7. However, due to the low total alkalinity of these low-EC water sources, its pH should drop when fertilizers, containing some acid residues, are added.
Apart from the limestone pebble treatment, the alkalinity of feeding water can also be increased by using soluble hydroxides such as potassium hydroxide (KOH) or calcium hydroxide (Ca(OH)2). However, the dosage of these products should be accurately calculated and applied to lift the total alkalinity and pH to higher levels. Water with a high total alkalinity needs to be treated with acid to lower its alkalinity before fertilizers can be added.
Harmful micro-organisms in water
The levels of plant pathogens are usually very low in water from boreholes. When using water from rivers, the incidence of plant pathogenic organisms is much higher. Due to informal settlements and urbanization, water sources that were relatively free from plant pathogens a few years ago, might have deteriorated, needing sterilization before it can be safely used for soil-less crop production.
Several laboratories do bacterial counts. As indication of the total number of bacteria in the water (good and bad), they determine the number of ‘coliforms per 100 ml’. Irrigation water should contain less than 1000 coliforms per 100 ml water, but a more reliable norm for the quality of the water is to measure the number of harmful bacteria such as Escherichia coli and Salmonella.
These bacteria should not be present in the water used for overhead irrigation systems (<1 per 100 ml). With a drip irrigation system, the norm is <10 per 100 ml. Where water is needed to rinse vegetable products, it should be municipality standard and fit for human consumption. According to SANS (2017), this means that the E. coli and Salmonella counts should be <1 per 100 ml water.
Apart from chlorination, as used by municipalities and some growers, or hydrogen peroxide, as used by some lettuce and blueberry growers, other sterilization options are also available. Due to the importance to have nutrient solutions sterilized before it is reused in closed production systems, several alternative sterilization options will be discussed in the next edition of Undercover Farming.
By: Dr NJJ (Nic) Combrink of the Horticultural Faculty, University of Stellenbosch. Readers who wish to obtain his very valuable updated manual on Nutrient Solution Management, may contact him at njjc@sun.co.za

